
Hardening Process and the Optimization of CO2 Levels in the Hardening Chamber
The curing of concrete blocks is an important process that determines the strength, durability and appearance of the material. The curing of the concrete blocks depends heavily on the ambient conditions. This is why modern hardening chambers have been developed in recent years, which permit the curing of concrete blocks in a controlled environment. This report looks at the various methods of curing concrete blocks. The pros and cons of the individual methods as well as the effects on the product appearance of the concrete blocks are shown and the application of CO2 in the curing environment is discussed.
When comparing the most important curing processes for concrete blocks, we consciously differentiate between a curing process and a drying process. The main difference between drying and curing is that drying only reduces the water content of the concrete block paver, while curing increases its strength, resistance and quality of appearance.
Drying in open rack systems
The simplest and least costly method of curing concrete blocks is drying them in an open rack system in the production hall. With this method, the concrete blocks are placed in a rack system after demoulding and left there for one or more days. The advantage of this method is the low acquisition cost. All that is required is a rack system installed in the production hall. The disadvantage of this method is the uneven drying of the concrete blocks, which is influenced by air temperature, air flow behaviour and the time of year. In winter, hardening may not be sufficient and the concrete blocks may not achieve the desired strength. Colour and strengths differences due to the location in the rack system and the surface evaporation due to draughts are the main reasons why this system is not often used today.
Drying in insulated curing chambers with air recirculation
Although insulation may improve moisture retention, we still speak of drying and not hardening, as there is no control over the relative humidity. Drying concrete blocks in an insulated curing chamber is an improvement on drying them in an open rack system. The curing chamber is insulated in such a way that the heat generated during the hydration of the concrete does not escape to the outside. Consequently, the concrete blocks dry faster in an insulated chamber. There are two large disadvantages of this method: 1. the drying chamber still depends on the heat generated by the hydration of the concrete. In the cold season, there may therefore still be delays and different colours compared to warmer seasons. 2. Moisture evaporated by the concrete cannot escape the chamber which leads to condensation, drips, spots and corrosion of the steel components.
Drying in heated curing chambers
In this method, external heat is added to overcome the disadvantages of drying in an insulated drying chamber with air recirculation. This may be achieved by a heating unit or by utilising the waste heat from another process. The advantage of this method is that the concrete blocks dry quickly and evenly. The chamber can be set to a constant temperature, so that the concrete blocks can be dried regardless of the time of year. The disadvantage of this method is that the concrete blocks are dehumidified by the heat. This can lead to quality losses, such as low wear resistance, poor frost/de-icing salt resistance, brittle corners and edges, and increased primary and secondary efflorescence.
Curing in heated hardening chambers with added moisture
Please note the switch from the word “Drying” to the word “Curing” in the subtitle. This important change is a step further to supply the hardening chamber with external moisture. This prevents problems caused by drying in heated chambers and significantly increases the hardness and density of the surface, corners and edges. Especially at high chamber temperatures, this is a necessary improvement that every modern chamber system should incorporate. The disadvantage, which is now gaining much more attention, is the lack of CO2 content in the hardening environment. The CO2 content in well-insulated hardening chambers is below the atmospheric level. Concrete absorbs CO2 from the air. The CO2 uptake is highest when fresh and decreases over time. This lowers the normal CO2 concentration in the chamber. However, the CO2 absorption goes far beyond the normal hardening time for many years.
Curing with heat, moisture and CO2 in the curing chamber
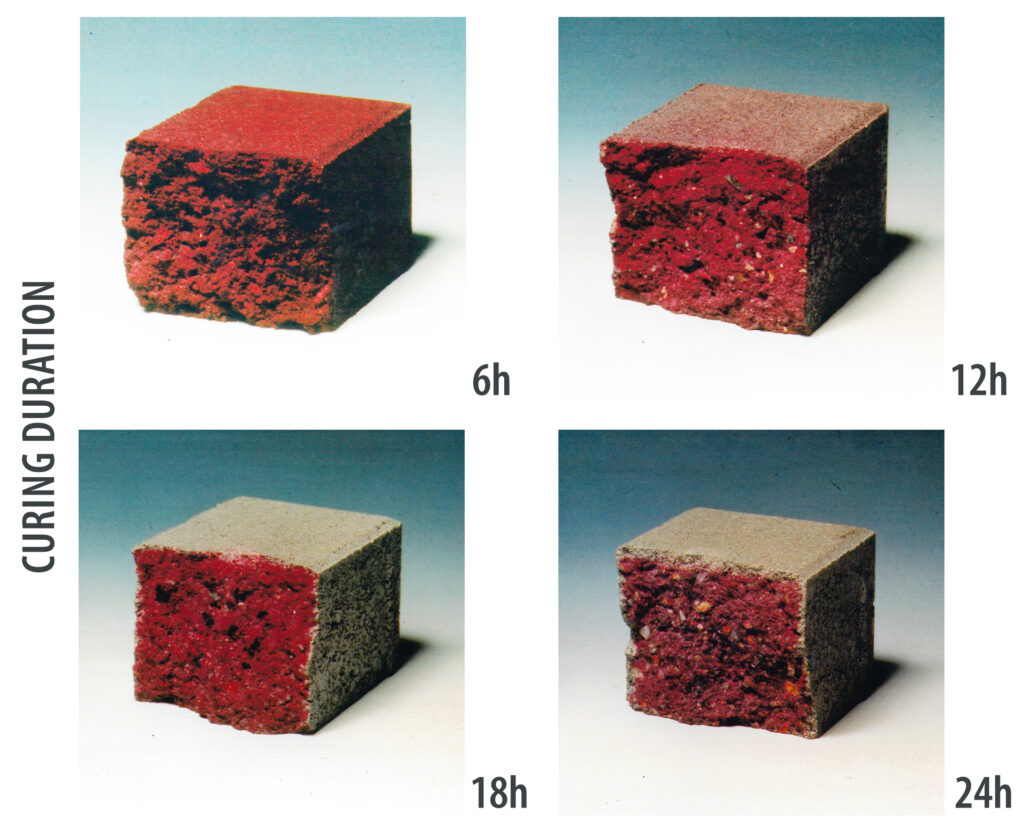
In heated curing chambers, with moisture and CO2 addition, the beneficial properties of the concrete block paving is intensified. The addition of CO2 raises its concentration in the chamber air. The carbonation depth can be up to 1 cm if the CO2 content, concrete mix-design and curing duration are optimised. A well-carbonated surface is a denser surface, which means that efflorescence is largely prevented and colour stability (permanence) is increased in the long term. The TestCube from Kraft Curing can be used to test various curing conditions and their effect on concrete products. Test trials in several concrete plants have shown that the addition of even small amounts of CO2, or hardening at a comparatively low CO2 concentration, has a positive effect on the products.

Influence of CO2 in a hardening environment
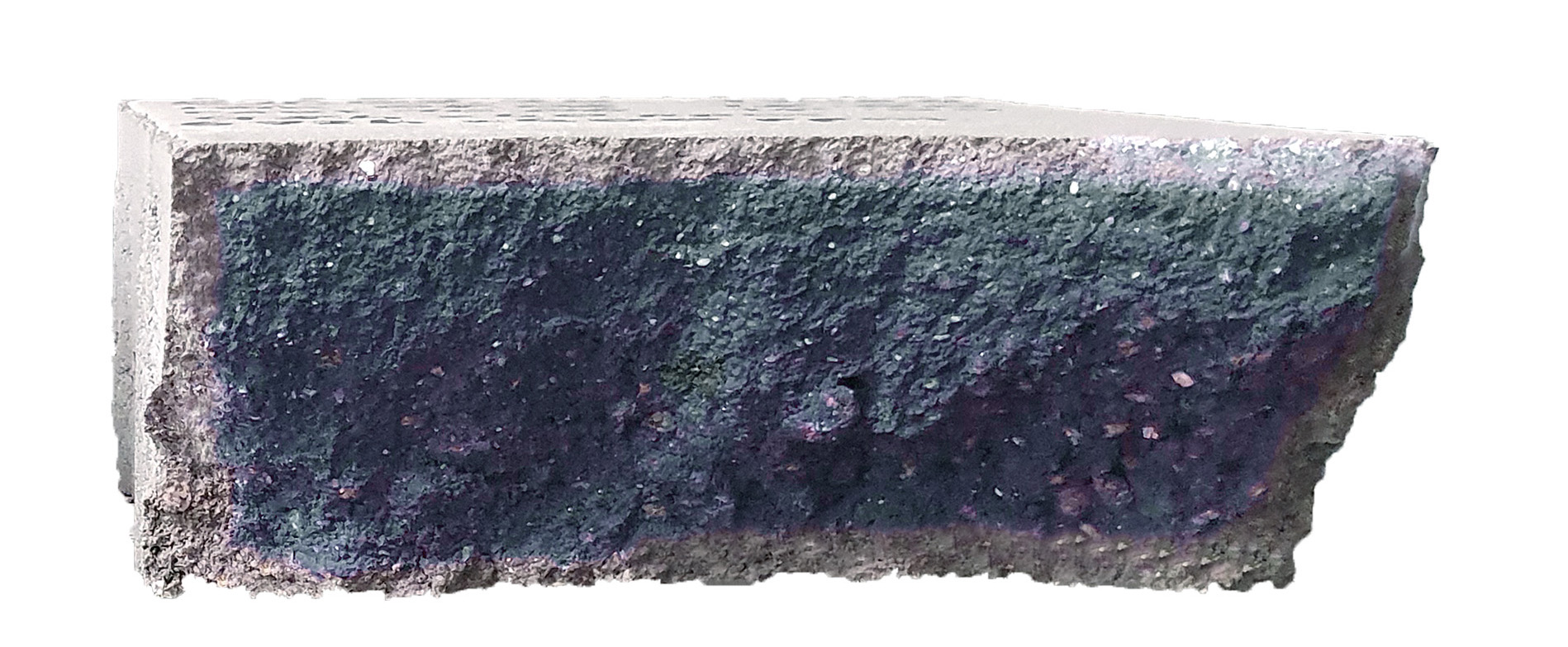
Increased quantities of CO2 increase the quality of the concrete blocks in the curing environment by increasing the hardness and strength of the concrete. Calcium hydroxide (Ca(OH)2) is formed during the hydration of the cement. This reacts with carbon dioxide (CO2) from the air to form calcium carbonate (CaCO3). The insoluble calcium carbonate forms a hard and resistant layer in the pores near the surface of the concrete. With higher quantities of carbon dioxide, more calcium carbonate is formed and the carbonation depth of the concrete increases. The colour stability (permanence) of the concrete therefore depends, among other things, on the amount of carbon dioxide added to the concrete during curing. The curing time in the chamber can increase the amount of CO2 absorption into the block, as the carbonation of the concrete block takes time. The %-diffusion depth decreases with the thickness of the concrete block. Hence, it is important that the concrete blocks can remain in the curing chamber for a sufficiently long time to facilitate carbonation. With a longer curing time in the chamber, the CO2 has more time to diffuse into the concrete block and react with the calcium hydroxide. This increases the amount of calcium carbonate in the concrete block and makes the concrete block harder, denser and stronger. Carbonation can be accomplished with appropriate modifications in a well-sealed curing chamber. The choice of chamber design determines the desired amount of CO2 in the hardening environment. During carbonation in a well-insulated hardening chamber, the CO2 is introduced into the hardening environment through a gas nozzle or a CO2 injector and mixed with the ambient air. Depending on the amount of CO2 in the environment, further measures are necessary to make the chamber safe for employees in the working environment.
Diffusion and condensation
Carbon dioxide can enter the concrete in a hardening chamber in various ways. Diffusion and condensation, for example, are both processes in which substances move from one place to another. The fundamental difference between the two processes is that diffusion is a passive phenomenon, while condensation is an active phenomenon. Diffusion is a process in which molecules or atoms move from one place to another to achieve equilibrium. Diffusion is caused by Brownian molecular motion, in which the molecules or atoms are constantly in random motion. During the diffusion of CO2 in concrete blocks, the CO2 molecules diffuse from the air through the surface of the concrete block into the interior. The diffusion rate depends on the density of the concrete, the CO2 concentration in the air and the temperature. Condensation is a process in which a gas is transformed into a liquid. Condensation occurs when the temperature of the gas falls below the dew point. The water vapour from the air condenses in the chamber, CO2 can be found as an accompanying substance in the condensate. From a purely logical point of view: The higher the CO2 concentration in the chamber air, the higher the proportion of CO2 in the water condensate. The humid layer has a higher diffusion rate than the dry concrete, which increases the diffusion rate of CO2 into the concrete block. In detail, the transport of CO2 in concrete blocks by diffusion and condensation can be explained as follows.
Diffusion
CO2 molecules from the air diffuse through the surface of the concrete block into the interior. The diffusion rate depends on the thickness and composition of the concrete block, the CO2 concentration in the air and the temperature.
Condensation
CO2, as a gas in the air together with water vapour, condenses to water on the surface of the concrete. The water forms a moist layer on the surface of the concrete block. The moist layer has a higher diffusion rate than the dry concrete block.
Condensation occurs in a concrete block plant when the fresh products enter the curing chamber from the production hall. The fresh products have a temperature of 15 °C, for example, and pass through an opening into a curing chamber with 35 °C and 95 % relative air humidity. Relative humidity is a measure of the proportion of water vapour in the air. At 100 % relative humidity, the air is saturated with water vapour and no further water vapour can be absorbed. When the fresh concrete products enter the curing chamber from the production hall, there is a large temperature difference between the products and the ambient air in the curing chamber. The products have a temperature of approx. 15 °C, the ambient air in the curing chamber has a temperature of 35 °C. This temperature difference causes the temperature of the air that comes into contact with the surface of the concrete to drop.
The relative air humidity in the curing chamber is 95 %. This means that the air in the curing chamber is already almost saturated with water vapour. When the temperature of the air on the surface drops, the dew point of the air also drops. The dew point is the temperature at which the water vapour condenses. The combination of diffusion and condensation leads to rapid and even carbonation of the concrete surface.
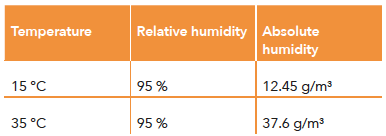
The relative air humidity indicates how much water vapour is present in the air compared to the maximum amount of water vapour that the air can absorb at the respective temperature. At a relative humidity of 95 %, the air is already almost saturated with water vapour.
• At a temperature of 15 °C, 1 m3 of air with a relative air humidity of 95 % therefore has an absolute humidity of 12.45 g/m3.
• At a temperature of 35 °C, 1 m3 of air with a relative air humidity of 95 % therefore has an absolute humidity of 37.6 g/m3.
The comparison shows that 1 m3 of air at a temperature of 35 °C can absorb significantly more water than at a temperature of 15 °C. In the example of the concrete block plant, the colder products enter the chamber and the almost completely saturated air on the surface of the products can no longer absorb the amount of humidity, which leads to condensation forming on the product.
CO2 levels and their influence on the human body
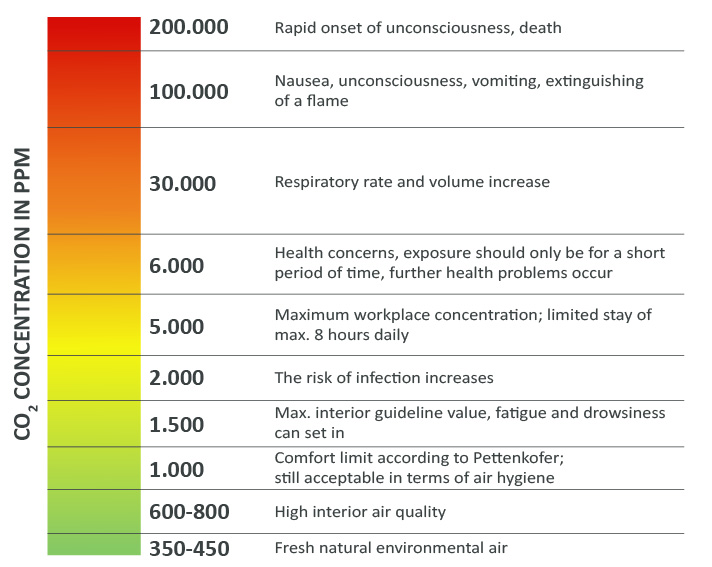
Carbon dioxide (CO2) is a natural component of the air. The normal concentration of CO2 in the Earth’s atmosphere is around 400 ppm (parts per million), i.e. around 0.04%. The Federal Environmental Agency recommends letting fresh air from outside into the room as soon as a value of 1,000 ppm CO2 is exceeded. The so-called maximum workplace concentration in enclosed spaces, also known as the MAK value for short, corresponds to just under 5,000 ppm — and that for a stay of 8 hours. This is to make the following values a little more tangible. At a CO2 concentration of around 1,000 ppm, you may experience mild symptoms such as headaches, tiredness and difficulty concentrating. At a CO2 concentration of around 5,000 ppm, symptoms such as dizziness, nausea, vomiting and breathing difficulties can occur. A CO2 concentration of over 10,000 ppm can lead to unconsciousness and death. The effects on the body increase with the length of stay.
The workplace next to a chamber with an increased CO2 content, depending on the concentration, should be treated with caution, as this can lead to health problems. However, the risk of permanent exposure to high CO2 concentrations in the hall is low, as the CO2 content in the hall is usually at the level of the normal ambient air. The installation of suitable measuring devices and warning systems is essential in a concrete block plant where products are cured in a chamber with a high CO2 concentration to protect the health of employees.
A vision for the future
Curing concrete with CO2 is a promising process. This is not only good for improving the quality of the stone products, but also for binding CO2 from the atmosphere and thus helping to combat climate change. High CO2 levels in the curing environment increase the hardness and strength of the concrete, increase colour stability (permanence) and help with the depth of carbonation, which can significantly reduce the susceptibility to efflorescence. As things stand at present, however, hardening with CO2 must still be questioned. The reasons for this are:
• The availability of recycled CO2 is still limited. Recycled CO2 is obtained from industrial processes in which CO2 is produced as a by-product. The capacities of these processes are not yet sufficient to meet the demand for recycled CO2 for hardening concrete blocks.
• The filter and collection technologies for recycled CO2 must be significantly improved. Recycled CO2 often still contains impurities that can have a negative effect. Consequently, filter and collection technologies need to be developed further so that they can effectively remove these contaminants.
• The question of transport must be clarified. Recycled CO2 is generally used close to the sources where it is produced. It makes no sense to transport CO2 over long distances to the concrete block plants. The question of transport must therefore be clarified so that recycled CO2 can also be used in regions with low CO2 availability. Despite these challenges, hardening with CO2 is a promising option for the future. If the availability of recycled CO2, the filter and collection technologies and the transport issue can be solved, it will be possible to produce concrete blocks that absorb significantly more CO2 than before.
This would make an important contribution to combating climate change and help to further improve stone quality.
Summary
The curing of concrete blocks in modern curing chambers has numerous advantages over drying in a rack system in the hall. The curing chamber guarantees more uniform curing, regardless of the time of year. Hardening can be accelerated by applying heat. Reinforced carbonation is the latest method for hardening concrete blocks and offers the best properties in terms of hardness, strength and durability.
chamber
The current CO2 values in the curing chambers are too low to achieve maximum CO2 absorption by the concrete blocks. Measurements on previous chamber systems show that the potential for CO2 absorption by concrete blocks is much higher than previously assumed. With an optimum CO2 concentration and corresponding curing time in the chamber, the CO2 absorption of the concrete block can be significantly increased in the future. CO2 curing is a process with great potential. The carbonation depth can be significantly improved with optimised CO2 contents and recipes. Furthermore, efflorescence should no longer be a problem with this process. It is without question an interesting alternative to conventional hardening processes. Research and testing is already underway in many areas.










